





























|
 March/April, 1998 Volume XII Number 11
March/April, 1998 Volume XII Number 11
Breast cancer, abortion, and the pill
 In addition to moral concerns, the "pill" and other chemical means of birth control raise health issues for women. In this interview Dr. Chris Kahlenborn talks about the "pill," abortion, and the breast cancer risk for those taking it.
In addition to moral concerns, the "pill" and other chemical means of birth control raise health issues for women. In this interview Dr. Chris Kahlenborn talks about the "pill," abortion, and the breast cancer risk for those taking it.
Q-: What is an oral contraceptive?
An oral contraceptive is usually a combination of a synthetic estrogen and progestin (i.e., the two major types of female hormones) which women take for 21 days out of a 28 day cycle. They work by suppressing, but not eliminating ovulation, thickening cervical mucus and by changing the lining of the uterus.
Q-: Is there any evidence that the OCP causes breast cancer in animals?
Yes. Concerns were raised in 1972 when it was noted that an oral contraceptive pill containing the artificial hormones mestranol and norethynodrel appeared to cause a case of metastatic breast cancer in a female rhesus monkey [252]. This was especially worrisome since rhesus monkeys rarely develop breast cancer-until that time only three cases of breast cancer in the rhesus monkey were known of. Although some argued that this was simply a "chance finding" concern grew further when it was noted that both beagles and rodents developed breast cancer when exposed to the hormones contained within today's OCPs.
Q-: How might OCPs cause breast cancer in humans?
In 1989, Anderson et al., published a classic paper regarding the influence of the OCPs on the rate of breast cell division. They found that nulliparous women (i.e., women who have not had children yet) who took OCPs had a significantly higher rate of breast cell division than nulliparous women who did not take them. This was especially important since it is known that cells which divide more rapidly are more likely to become cancerous.
Q-: Do oral contraceptives cause an early abortion and if so, could this also be playing a role in the increased risk of breast cancer?
It is conservatively estimated that a woman who takes the oral contraceptive pill (OCP) will have at least one abortion for every year that she is on it. Both pro-life and pro-abortion groups admit this, with the latter doing so publicly in front of the Supreme Court in 1989 (The New York Times). Induced abortion before a woman's first-term pregnancy has been noted to increase a woman's risk of breast cancer by 50%. Could an abortion within the first week of conception have a deleterious effect as concerns breast cancer? The hormonal physiology of early pregnancy is difficult to measure but Stewart et al. have shown that estradiol and progesterone levels (i.e. the female hormones) start to rise above baseline levels within four days of conception, thus prior to implantation and before B-hCG levels begin to rise. Could this early "hormonal blow" be playing a role? To this author's knowledge, no one has asked or studied this question.
Q-: Can you give a brief history of the studies which showed a link between OCPs and breast cancer?
In 1981, Pike et al., found that women who took OCPs before their first term pregnancy had a 2.4 fold increased risk of developing breast cancer before age 32. This startled the research world and led to additional studies, including a very large American trial called the CASH study (i.e., Cancer And Steroid Hormone study). In 1993, the CASH study showed that women under 44 years of age had a 40% increased risk in breast cancer, which was statistically significant in the 35-44 age group.
Later in England, Chilvers et. al. published the results of another large study called the United Kingdom National Study. She showed that young women under the age of 36 who had used oral contraceptives for at least 4 years before their first term pregnancy had at least a 44% increased risk in breast cancer. The last large study was performed in 1995 by Brinton et. al. It showed a 41% increased (raw relative) risk for women who used OCPs for more than 6 months prior to full term pregnancy.
Q-: If the major studies showed the risks that you have just mentioned, then why do doctors and pharmacists fail to inform their patients of those risks?
That is a good question. Unfortunately, it does not help when major journals and major medical associations fail to stress the dangers of early oral contraceptive use. Part of the problem is that because the OCP/breast cancer debate is complicated, most lay people have to rely on what "the experts" tell them.
A good example of this occurred recently in the study written in condensed version in The Lancet and in complete form in Contraception. This was and remains the largest meta-analysis (i.e. a synthesis of all the major studies done in a particular field, concluding in an overall risk for the pooled studies) regarding the studies of OCPs and breast cancer. Researchers from around the world studied and combined the data from 54 studies, involving 25 countries and 53,297 women who had breast cancer. It concluded that, "Women who are currently using combined oral contraceptives or have used them in the past 10 years are at a slightly increased risk of having breast cancer diagnosed, although the additional cancers tend to be localized to the breast. There is no evidence of an increase in the risk of having breast cancer diagnosed 10 or more years after cessation of use..."
Unfortunately, this study is known more for what it did say, than what it did not say! There were several major weaknesses with the study. The main weakness was the failure to report any evidence of what the pooled risk of oral contraceptive use before first term pregnancy was in women less than 45 years old. Another major weakness is that the Oxford study pooled data from studies which studied women with breast cancer from the early and mid 1970s.
Q-: Why are the items mentioned in the previous answers considered "weaknesses"?
A woman's breast is especially sensitive to carcinogenic influence (i.e., cancer producing influence) before she has her first child since the breast undergoes a maturing process throughout a woman's first pregnancy. By failing to measure the effect of OCP use before a woman's first term pregnancy (FTP), the Oxford study failed to give data on the one group of women who are most likely to get breast cancer from oral contraceptives-those who use them before their first term pregnancy (FTP).
The second weakness is that the Oxford study used data from older studies taking some of its data from the mid and early 1970s. This does not leave a long enough latent period. A latent period is the time between a suspected risk factor (e.g., early OCP use) and the cancer which it increases (e.g., breast cancer). Often the latent period between a risk factor and a cancer is 15 to 20 years or more. Women in the U.S. began taking OCPs in the 1960s but they only began taking them for longer periods at early ages in the 1970s, and thus only studies which have data from the 1980s and 1990s or beyond would allow a long enough latent period to pick up the influence of early OCP use.
Q-: What do the four largest studies--which take the bulk of their data after 1980--state regarding women who used OCPs prior to first term pregnancy (FTP)?
The following table shows the results of the four largest studies which examined women under age 45 and which took most of their data after 1980.
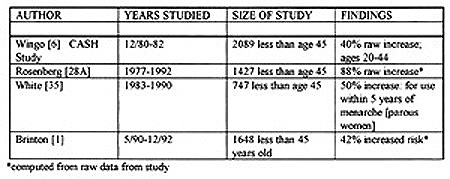
Q-: What do these studies mean?
In summary the four largest studies of women under the age of 45 all show at least a 40% increased risk for women who took OCPs prior to their FTP or within five years of menarche. Two studies (Rosenberg and Brinton) did not list a formal risk but it was calculated from their raw data.
Q-: Why is it important to study women who are under age 45?
Women who are under age 45 are more likely to have used OCPs prior to first birth than women over 45. For example a 55-year old women who had breast cancer in 1990 would have been very unlikely to have taken the OCP for a significant period of time prior to first birth since OCPs were just coming to the U.S. in the early 1960s, and the cited woman would have been in her late 20s at this time.
Q-: Has anyone done a meta-analysis that looked at the question of risk in women under age 45 who had taken OCPs prior to FTP?
Yes. Two different researchers have addressed this question. Thomas et al., in 1991, found that women who took OCPs for extended periods of time prior to FTP had a 42% increased risk. A more refined meta-analysis by Romieu et, al., in 1993 who restricted her analysis to those studies done after 1980, showed that women under age 45 who had taken OCPs for four or more years prior to FTP had a 72% increased incidence of breast cancer.
Q-: Can you give an overall statement regarding early OCP use and breast cancer?
Yes. If a woman takes the oral contraceptive pill before her first born child she suffers a 40% increased risk of breast cancer and if she takes OCPs for four years or more prior to her first baby, she suffers at least a 72% increased risk in breast cancer.
Q-: Are any other groups of women at high risk?
Yes, women who take OCPs for a prolonged periods of time (i.e., four years or more) [8,26,34], those who use them after age 25, and nulliparous women who use them for a prolonged time (i.e., four or more years) all seem to be at increased risk with individual studies ranging from 40% to over 200% increased risk.
Q-: The studies you alluded to involved women who were less than 45 years old from data taken after 1980. What will happen to the risk for these women as they grow older?
No one knows. It would be wise to learn from history. In the late 1940s an artificial female hormone named DES (Diethylstilbestrol) was given to women to prevent miscarriages. For over 25 years researchers maintained that DES did not increase the risk of breast cancer in women who took it. Finally, in the 1980s, it was discovered that DES increased breast cancer by about 35%-especially in older women. The truth is, no one knows how dangerous OCPs will be in women as they grow older.
Q-: It has been noted that OCPs reduce the rate of uterine and ovarian cancer--is this true?
Yes, it is true. However it must be noted that OCPs also increase the risk of cervical and liver cancer. In addition, more women get breast cancer in the U.S., than all of the other alluded to cancers combined, making this the most dangerous risk in western countries. OCPs may be particularly risky in Asian and African countries where liver cancer is far
more prevalent than in western countries.
Q-: What about the risk of progestin containing contraceptives such as "the mini-pill," or long-acting progestins such as Norplant or Depo-Provera?
Skegg et al., pooled the data of the WHO (World Health Organization) and New Zealand studies which were the two largest studies that looked at women who took Depo-Provera (active ingredient is DMPA: depo-medroxyprogesterone acetate) for long periods of time. He found that women who had taken DMPA for between two and three years before age 25 had a 310% statistically significant risk of getting breast cancer {RR=4.1: (1.6-10.90} while women who had taken DMPA for more than 3 years prior to age 25 had at a 190% increased risk that was also significant {2.9: (1.2-7.1)}. The risks for long-term Norplant use in young women have not been extensively studied but there are no good reasons to believe that they would be different from that of Depo-Provera.
Q-: How can I verify all of this information?
Go to your nearest medical library--nearly every hospital has one--and ask the librarian to help you look up the medical sources you are interested in.
Interested in doing your own research? Dr. Kahlenborn has drawn from the following resources:
1. Kahlenborn C. How do the pill and other contraceptives work? Life Advocate. July 1997; 20-25.
2. Alderson Reporting Company. Transcripts of oral arguments before court on abortion case. New York Times. April 27, 1989; B12.
3. Brind J, Chinchilli M, et al. Induced abortion as an independent risk factor for breast cancer: a comprehensive review and meta-analysis. J. Epi. and Comm. Health. 10/ 1996; 50: 481-496.
4. Stewart DR, Overstreet JW, et al. Enhanced ovarian steroid secretion before implantation in early human pregnancy. J. of Endo. and Met. 1993; 76: 1470-1476.
5. Kirschstein RL et al. Infiltrating duct carcinoma of the mammary gland of a Rhesus monkey after administration of an oral contraceptive: a preliminary report. JNCI; 48: 551-553.
6. Geil et al. FDA studies of estrogen, progestogens, and estrogen/progesterone combinations in the dog and monkey. J Tox. Env. Health. 3: 1979.
7. Shubik P. Oral contraceptives and breast cancer: laboratory evidence. In: Interpretation of Negative Epidemiological Evidence for Carcinogenicity. IARC Sci. Publ. 1985: 65; 33.
8. Kahn RH et al. Effect of long-term treatment with norethynodrel on A/J and C3H/Hej mice. Endocrinology 1969; 84: 661.
9. Weisburger JH et al. Reduction in Carcinogen Induced Breast Cancer in rats by an anti-fertility drug. Life Sci. 1968; 7: 259.
10. Welsch CW et al. 17B-Estradiol and enovid mammary tumorogenesis in C3H/HeJ female mice: counteraction by concurrent 2-bromo-ergo cryptine. Br. J. Cancer. 1977; 35: 322
11. Anderson T, Battersby S, et al. Oral contraceptive use influences resting breast proliferation. Hum. Pathol. 1989; 20: 1139-1144.
12. Pike MC, Henderson BE, et al. Oral contraceptive use and early abortion as risk factors for breast cancer in young women. British Journal of Cancer. 1981; 43: 72-76.
13. Wingo PA, Lee NC, et al. Age-specific differences in the relationship between oral contraceptives use and breast cancer. Cancer (supplement). 1993; 71: 1506-17
14. Chilvers C, McPherson K, et al. Oral contraceptive use and breast cancer risk in young women {UK National Case-Control Study Group}. The Lancet. May 6, 1989: 973-982.
15. Brinton LA, Daling JR et al. Oral contraceptives and breast cancer risk among younger women. JNCI. 6/7/1995; 87: 827-35.
16. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. The Lancet 1996; 347: 1713-1727.
17. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: further results. Contraception. 1996; 34: S1-S106.
18. Rosenberg L, Palmer JR, et al. Case-control study of oral contraceptive use and risk of breast cancer. American Journal of Epidemiology. 1996; 143: 25-37.
19. White E, Malone K, Weiss N, Daling J. Breast cancer among young U.S. women in relation to oral contraceptive use. JNCI. 1994; 86: 505-514.
20. Thomas DB. Oral contraceptives and breast cancer: review of the epidemiological literature. Contraception. 1991; 43: 597-642
21. Romieu I, Berlin J, et al. Oral contraceptives and breast cancer. Cancer. 1990; 66: 2253-2263
22. Colton T, Greenberg ER, et al. Breast cancer in mothers prescribed diethylstilbestrol in pregnancy. JAMA, 1993; 269: 2096-3000.
23. Rookus MA, Leeuwen FE. Oral contraceptives and risk of breast cancer in women ages 20-54 years. The Lancet. 1994; 344: 844-851.
24. Weinstein A, Mahoney M, et al. Breast cancer risk and oral contraceptive use: results from a large case-control study. Epidemiology. 1991; 2: 353-358.
25. Palmer J, Rosenberg L, et al. Oral contraceptives use and breast cancer risk among African-American women. Cancer Causes and Control. 1995; 6: 321-331.
26. Thomas DB, Noonan EA. Breast cancer and combined oral contraceptives: results from a multinational study [The WHO collaborative study of Neoplasia and steroid contraceptives]. 1990; 61: 110-119.
27. Wang Q, Ross R, et al. A case-control study of breast cancer in Tianjin, China. Cancer Epidemiology. 1992; 1: 435-439.
28. Miller D, Rosenberg L, et al. Breast cancer before age 45 and oral contraceptive use: new findings. American Journal of Epidemiology. 1989; 129: 269-279.
29. Thomas DB et al. Oral contraceptives and invasive adenocarcinomas and adenosquamos carcinomas of the uterine cervix. American J. of Epidemiology. 1996; 144: 281-289.
30. Kenya PR. Oral contraceptive use and liver tumours: a review. East African Medical Journal. 1990. 67:146-153.
31. Ebeling K. et al. Use of oral contraceptives and risk of invasive cervical cancer in previously screened women. Int J. of Cancer. 1987; 39: 427-430.
32. Skegg DCG, Noonan EA, et al. Depot medroxyprogesterone acetate and breast cancer [A pooled analysis of the World Health Organization and New Zealand studies]. 1995; JAMA: 799-804.
OTHER
COVER
STORY
ARTICALS
Making Baby
Breast cancer, abortion, and the pill
The Reproduction Revolution

|
|
 In addition to moral concerns, the "pill" and other chemical means of birth control raise health issues for women. In this interview Dr. Chris Kahlenborn talks about the "pill," abortion, and the breast cancer risk for those taking it.
In addition to moral concerns, the "pill" and other chemical means of birth control raise health issues for women. In this interview Dr. Chris Kahlenborn talks about the "pill," abortion, and the breast cancer risk for those taking it.


